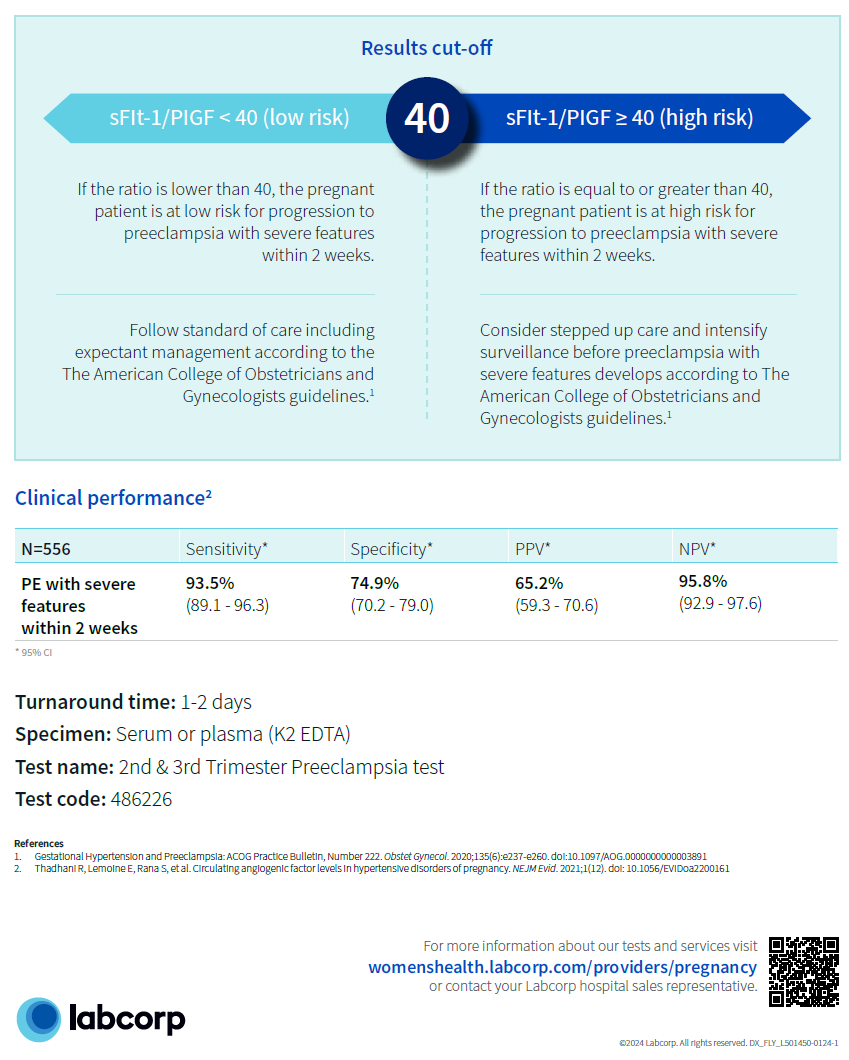
FDA-cleared second and third trimester preeclampsia test developed by ThermoFisher looks at risks from hypertension to severe features of preeclampsia
The blood test, with a turnaround time of 1-2 days, is designed for hospitalized patients between 23-35 weeks of gestation and works by analyzing the ratio of biochemical markers, with a ratio number of 40 or more indicating high risk for preeclampsia and under 40 indicating low risk. This test is not a standalone test and should be used as an additional tool to assess a patient’s risk with its performance characteristics based on the PRAECIS study.